V-MEISTER
Polysaccharide-Based Injectable Dermal Filler
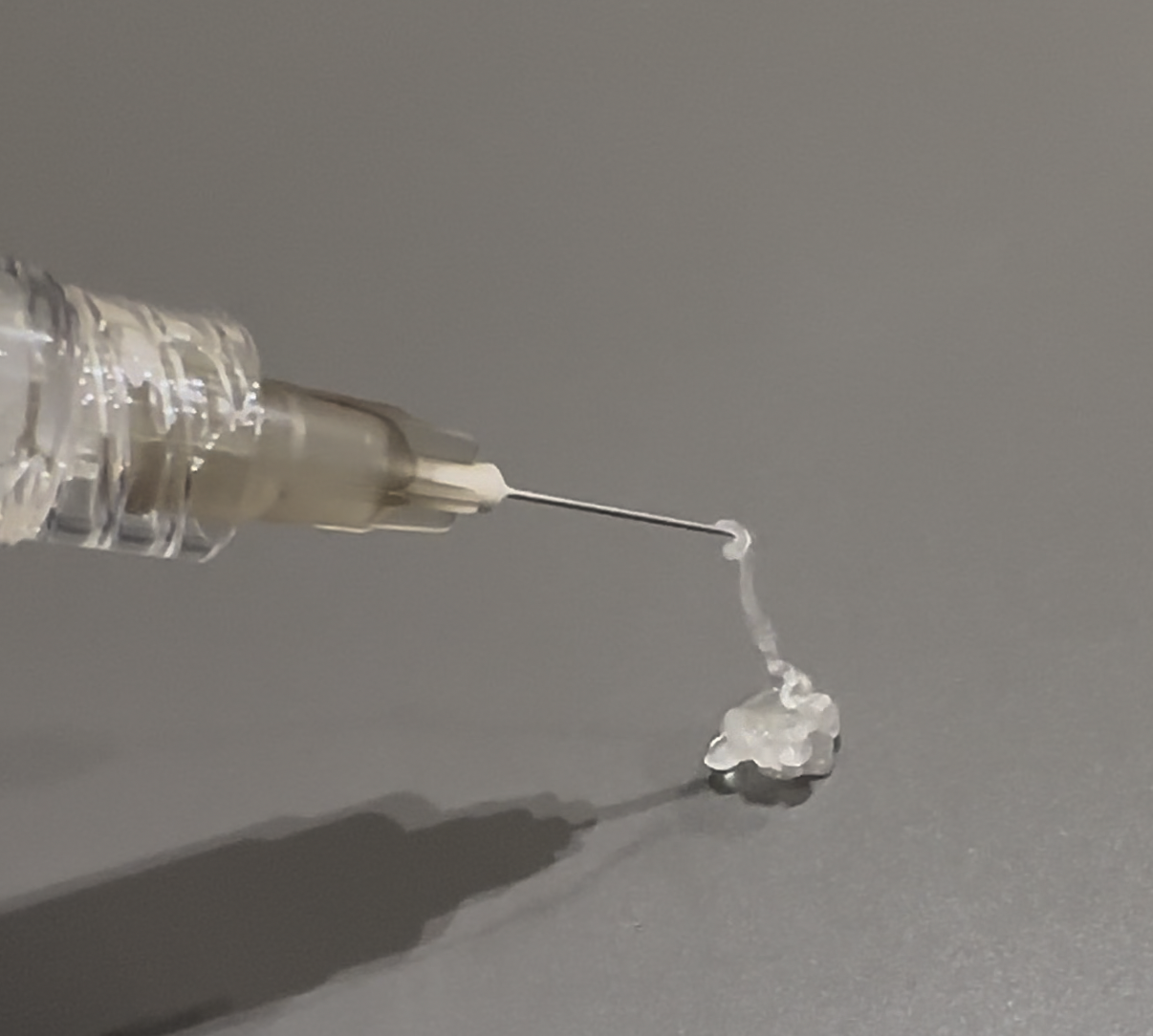
Product Overview
V-MEISTER is a premium polysaccharide-based injectable product designed as an advanced biostimulant. It is suitable for indications where neocollagenesis is desired. It is a unique material for use in penile, breast, and hip. It is the only soft-polymeric biostimulant in the market. Engineered to meet stringent regulatory and quality standards, V-MEISTER provides a reliable foundation for innovative medical applications.
Key Features
Proprietary Microspheric Polysaccharides – Ensuring superior tissue-like feel
Customizable Formulation – Designed for flexibility in medical device development. Different concentrations are available.
ISO 13485 Certified Manufacturing – Ensuring traceability and compliance with international standards.
Applications
V-MEISTER is specifically developed for use in:
Soft Tissue Augmentation – Neck, Face, Hands, Knee, Penis, Breast, and Hip.
Injectable Biomaterials – Facilitating advanced medical innovations.
Packaging & Delivery
1 mL Syringes – Ready to use in sterile condition.
Outer Packaging: Tyvek pouch for additional protection.
Sterilization
Steam sterilized at 121°C for 20 minutes.
Storage Conditions
Temperature: Store between 5°C – 30°C.
Humidity: Maintain low humidity conditions.
Precautions: Avoid exposure to high temperatures and direct sunlight.
Contact Us
For pricing and further information, please contact Meridian Biomaterials Co.
Email: info@meridianbio.tech
We are happy to assist with any questions regarding specifications, applications, and customization options.
